Classification of Pericyclic Reactions
Introduction
Most of
the organic reactions proceeds in step wise manner. However there are certain reactions, which
proceed in a single concerted step via formation of a cyclic transition state
involving π or σ electrons.
OR
Electron moves round a circle and
there are no positive or negative charges on any intermediates-indeed, there
are no intermediates at all, such type of reaction is called Pericyclic reactions.
eg. Diels-Alder reaction.
The energy for Pericyclic reaction is supplied by heat in
thermally induced reaction or by ultraviolet in a photo-induced reaction.
Pericyclic reactions are highly stereospecific. Thermal and photo-chemical
process gives different products but specific stereochemistry.
Pericyclic reactions
do not involve ionic or free radical intermediates and so solvents and reagents
have no effect on the source of reaction.
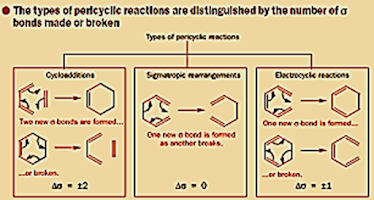
Types of Pericyclic
reactions:
The three most common types of Pericyclic reactions are
1)
Cycloaddition
reaction
2)
Electro
cyclic reaction
3)
Sigma
tropic reaction
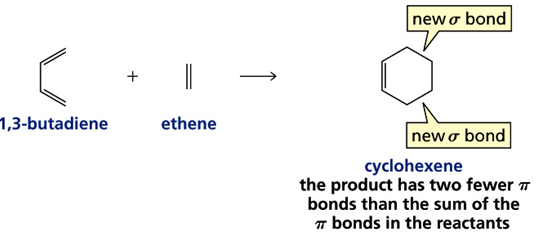
1) Cycloaddition Reactions
In these reactions two or more π electron system combines to form a ring. The reaction involve conversion of two π bonds into two sigma bonds (or reveres).
An example of cycloaddition reaction is well known as Diels-Alder reaction.
2)2)Electrocyclic Reactions
These are
reversible reaction in which a compound with conjugation double bond undergoes cyclization.
In this process two π electrons are used to form a σ-bond.
eg. 1,3,5 hexatriene
on heating gives 1,3 cycloheaxadiene
In the above illustration, an electrocyclic ring closer takes
place. The reverse of this process is an electrocyclic ring opening.
3) Sigmatropic Rearrangements
These
are concerted intermolecular rearrangements. In these rearrangements an atoms
(or group of atoms) shift from one position to another. The process involves
breaking of bond and forming of a new bond.
(But number of π bonds remains same)
eg. 3-methyl 1,5 hexadiene on heating gives 1,5
heptadiene.
Common example of sigmatropic reaction is Claisen rearrangement.
Types of Pericyclic reaction
Group Transfer Reaction: - a reaction in which one or more groups or atom transfer from one molecule. In this reaction both molecule are joined together by σ-bond.





No comments:
Post a Comment